Molecular Diagnostics Products
1. Altona Diagnostics
altona Diagnostics is a medical diagnostic company that develops and manufactures in-vitro diagnostic tests for the DNA based detection of pathogens such as viruses, bacteria or parasites. Headquartered in Hamburg-Altona, Germany, altona Diagnostics is privately owned and employs more than 300 people worldwide. The company has been in the molecular diagnostics business for over 20 years and is ISO 13485 certified.
All about SARS-CoV-2 testing!
Here you'll find a quick, on-the-fly guide about our SARS-CoV-2 RT-PCR testing: www.coronavirus-altona-dx.com
AltoStar® Real-Time PCR Reagents
The AltoStar® PCR kits are based on real- time PCR technology, utilizing polymerase chain reaction (PCR) for amplification of specified target sequences and target-specific probes in order to detect amplified DNA or RNA on the CFX96™ Deep Well Real-Time PCR Detection System (IVD, Bio-Rad).
RealStar® Real-Time PCR Reagents
All RealStar® PCR reagents are designed for quantification/qualititative detection and/or differentiation of pathogen-specific RNA/DNA in human matrix/matrices. A heterologous amplification system (Internal Control) is included to identify possible PCR inhibition. Please note the regulatory status of our three different product groups(CE/RUO/ASR).

The AltoStar® Automation System AM16 is a robotic pipetting workstation used for sample purification and PCR setup controlled by the AltoStar® Connect software. It is intended to be used in combination with the AltoStar® Purification Kit 1.5, the AltoStar® Internal Control 1.5 and altona Diagnostics PCR Kits for automated purification of nucleic acids and automated assay setup for in vitro diagnostic purposes.
2. Diatech Pharmacogenetics
Diatech Pharmacogenetics is the Italian leader in the development, production and commercialisation of pharmacogenetics tests for cancer precision medicine.Founded in 1996, Diatech Pharmacogenetics has sustained constant organic growth over the years and now owns more than 70% of the Italian molecular diagnostic market and it is rapidly growing worldwide; to date, more than 20.000 diagnostic tests have been performed using its solutions and every year Diatech Pharmacogenetics reinvests 20% of it revenues in R&D.Diatech Pharmacogenetics is able to take pharmacogenetics tests from idea to market on a variety of platforms, from real-time PCR to barcoded microarrays, mass-spectrometry and next generation sequencing, supplying all reagents, instruments, materials and support necessary. Its complete solutions guide diagnostic operators from sample extraction, quality control, molecular assay to bioinformatic analysis and report.
EASY LINE RT PCR KITS
The Easy® kits allow the qualitative detection of the main somatic mutations of the genes EGFR, KRAS, NRAS and BRAF by Real-Time PCR in association with a system of enrichment of the mutated allele.Each kit includes all the reagents necessary for the test and the positive controls of reaction.All the kits share the same thermal profile for the detection of the somatic mutations.
EasyPGX system workflow: from tissue to result in less than 3 hours.
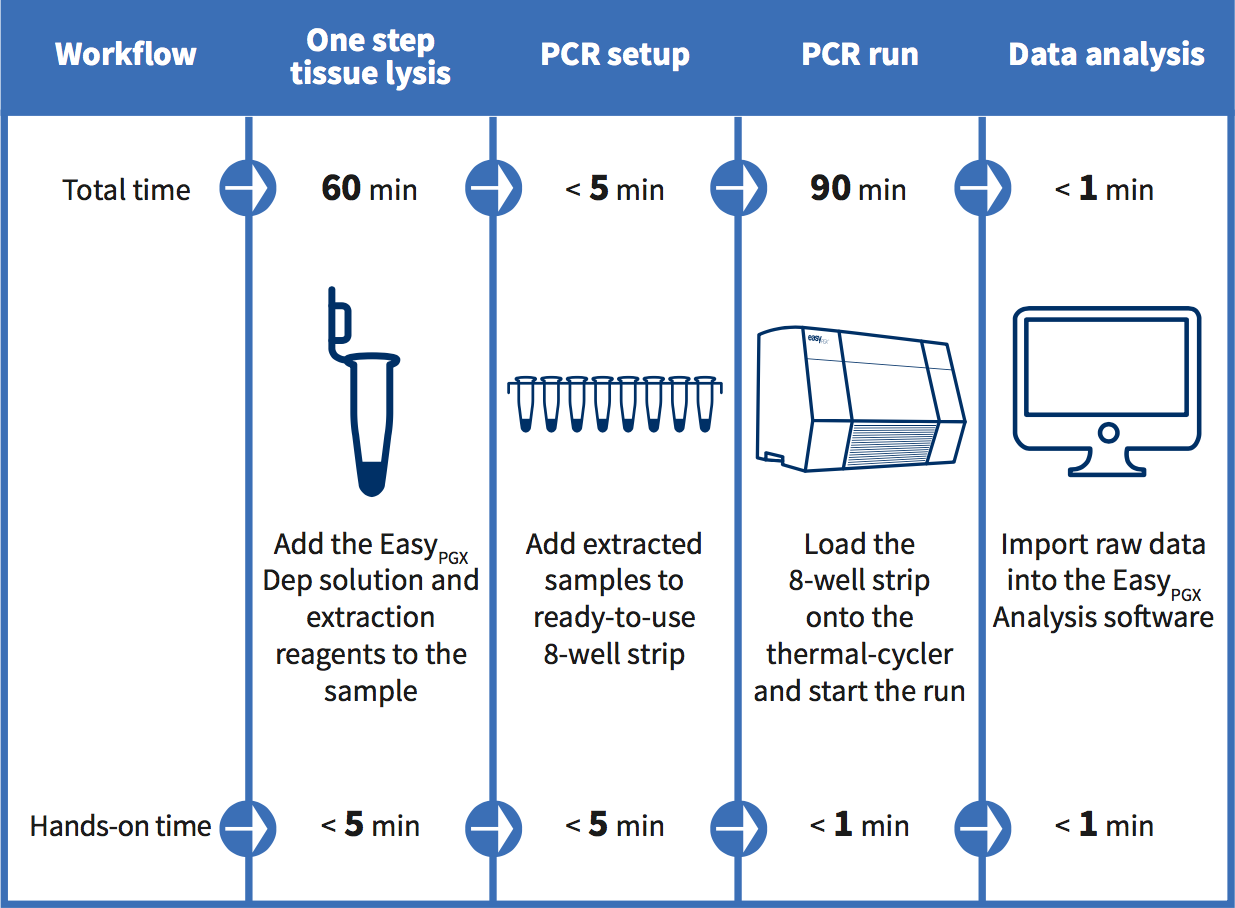
EasyPGX® product line – key features
Ready to use: reagents are delivered in 8-well strips preloaded with a complete master mix in a dry, room temperature and stable format.
Easy to use: no need for freezing, thawing or pipetting on ice and the few remaining pipetting steps minimize the risk of errors or contaminations.
High sensitivity: limit of detection as low as 0.5%.
Flexible sample requirement: low DNA input from a variety of sources, including FFPE and plasma.
Turnaround time: from tissue to result in less than 3 hours with only 10 minutes of hands-on time.
Quality assurance: manufactured under ISO 13485.
Regulatory: kits have been designed, developed and validated in accordance with the Directive 98/79/EC on in vitro diagnostic medical devices.
3. Certest (ViaSure) Biotech SL
CerTest Biotec, S.L. is an independent biotechnology company devoted to the development and manufacturing of IVD diagnostic products in human clinical field. The ViaSure brand is a complete Solution that helps to achieve a better diagnostic work flow.
The ViaSure product portfolio is having both Multiplex and Monoplex RT PCR kits suitable for various disease diagnostics


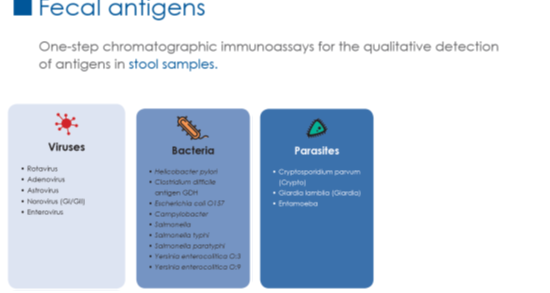
CerTest Rapid Tests
One step Immunochromatographic test





4. DNA Diagnostics
DNA Diagnostic A/S is a Danish biotech company founded in 1992, specialized in the development of PCR (Polymerase Chain Reaction) and qPCR (Quantitative Polymerase Chain Reaction), test kits for food safety, animal and human health care.
HemaVision
HemaVision® is a series of CE marked in vitro diagnostic (IVD) tests for fast and sensitive detection of chromosomal translocations associated with leukemia. The tests are 4 hours RT-qPCR screening tests, capable of analysing for up to 28 translocations and more than 145 clinical relevant translocation breakpoints in a single test.
- HemaVision-28Q
RT-qPCR HemaVision® kit for the screening and detection of 28 chromosome translocations with more than 145 clinically relevant chromosomal breakpoints in just 4 hours. Supplied in three types of qPCR tubes: WRP, WLP or FRP. Contain material for 12 tests. - HemaVision-7Q
RT-qPCR HemaVision® kit for the screening and detection of 7 chromosome translocations with more than 40 clinically relevant chromosomal breakpoints in just 4 hours. These 7 translocations are t(9,22) minor, major,and micro; t(1,19); t(12,21); inv16; t(15,17) S, V and L; t(8,21) and t(4,11). Supplied in tree types of qPCR tubes: WRP, WLP or CRP. Contain material for 12 tests.
5. Master Diagnostica
DNA FLOW
We offer a wide range of Molecular Diagnosis Systems for the simultaneous detection of panels of pathogens and markers, based on multiplex PCR or RT/PCR and subsequent hybridization using the DNA-FLOW technology. It is an innovative technology of automated flow-through reverse hybridization on a microarray of a porous membrane that contains specific oligonucleotides to each marker. The target DNAs go through the membrane by a vertical vacuum flow, which facilitates the interaction with their specific and complementary DNA probes immobilized on the porous membrane, in a three-dimensional environment, as opposed to the conventional dot-blot technology, in which hybridization occurs passively. As a result, the process speeds up and the sensitivity increases, reducing the reaction volumes and the total processing time from several hours to a few minutes.
Once the binding between the specific amplicons and their corresponding probes has occurred, the signal is visualized through an immunoenzymatic colorimetric reaction with Streptavidin−Alkaline Phosphatase and the NBT/BCIP chromogen, generating insoluble precipitates in the membrane in those positions in which there has been hybridization. The process is done automatically in the hybriSpot™ platforms and the results are analyzed with the hybriSoft™ software.

HYBRISPOT - HS 12

HYBRISPOT - HS 24
6. Patho Finder
PathoFinder is a privately owned molecular diagnostics company founded in 2004. The company focuses on the development and commercialization of comprehensive panels, with special emphasis on infectious diseases. PathoFinder's proprietary 2SmartFinder® technology enables the detection and differentiation of up to 24 viral, bacterial, fungal and parasitic pathogens in a single assay. PathoFinder is based in Maastricht, Netherlands
SINGLE TUBE MULTIPLEX AMPLIFICATION IN REAL TIME
The SmartFinder® technology is PathoFinder’s proprietary state-of-the-art multiplex PCR technology for real-time Molecular Diagnostics. It enables Single tube Multiplex Amplification in Real-Time (SMART) of up to 24 pathogens in a clinical sample.
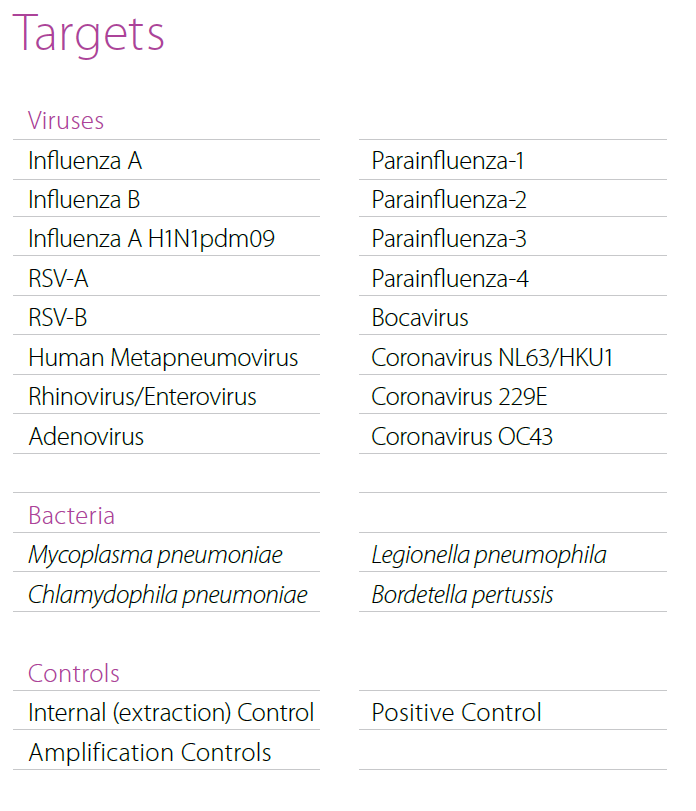
- RespiFinder® 2Smart
Multiplex real-time PCR for syndromic approach of respiratory tract infection diagnostics. Simultaneous detection of 20 viral and 4 bacterial pathogens. - GastroFinder® 2Smart
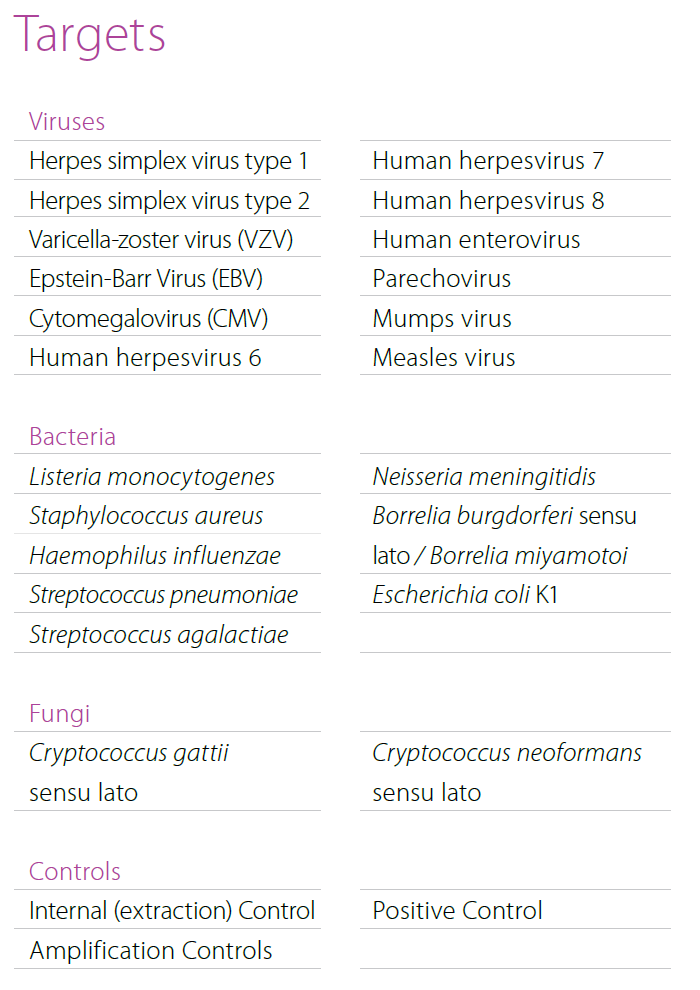
Multiplex real-time PCR for syndromic approach of gastrointestinal tract infection diagnostics. Simultaneous detection of 9 bacterial, 4 parasitic and 5 viral pathogens. - MeningoFinder® 2Smart
Multiplex real-time PCR for syndromic approach of central nervous system infection diagnostics. Simultaneous detection of 12 viral, 8 bacterial and 2 fungal pathogens. - D. STD-Finder® 2Smart
Multiplex real-time PCR for syndromic approach of sexually transmitted diseases diagnostics. Simultaneous detection of 5 bacterial, 1 parasitic and 2 viral pathogens.
RESPIFINDER
GASTROFINDER
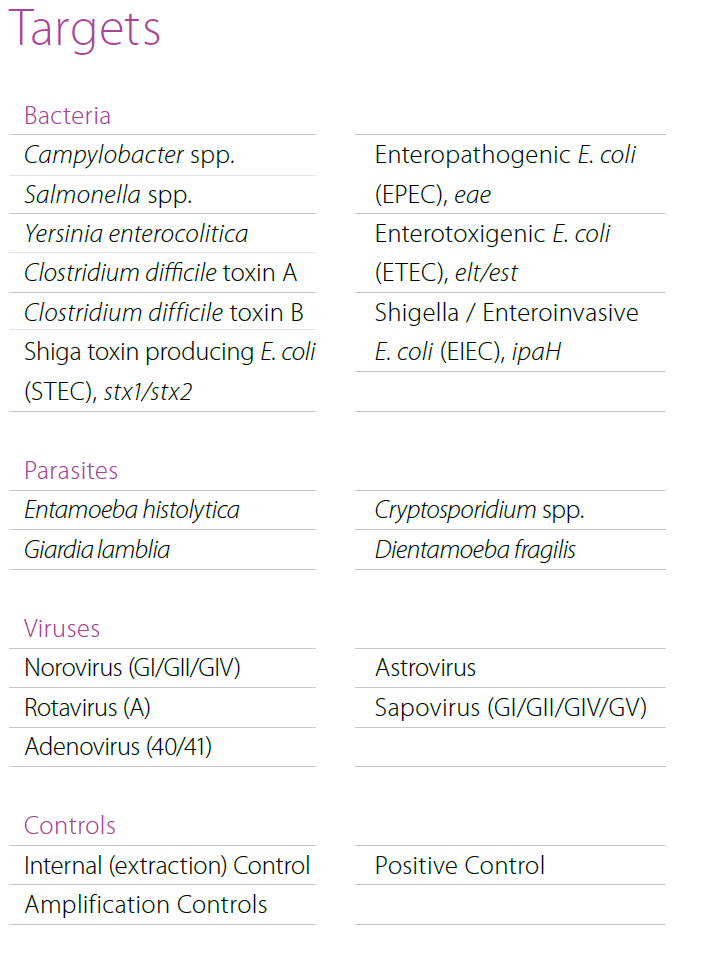
MENINGOFINDER